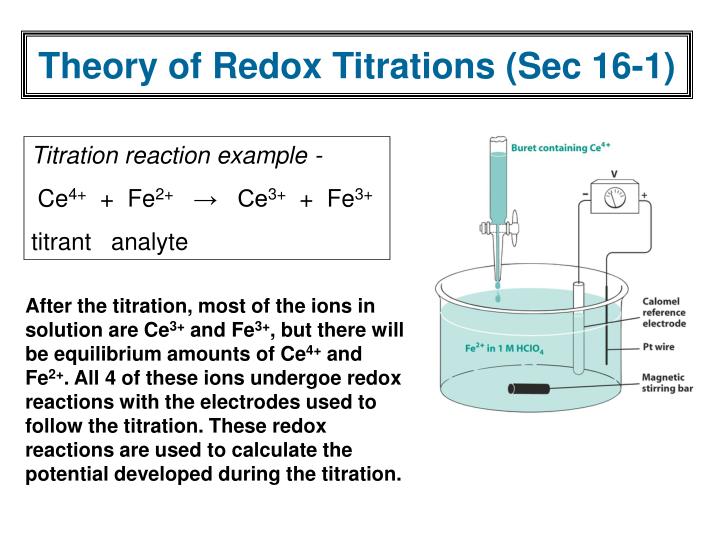
Titration Concentration Formula . Suppose that a titration is performed and \(20.70 \: Titration is a technique of determining the concentration of unknown solution by using a solution of known concentration. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). \ce{naoh}\) is required to reach the end point when titrated. \ce{naoh}\) is required to reach the end point when titrated. In many cases it is not a simple matter to obtain a pure substance,. The known solution (titrate) is added in. Suppose that a titration is performed and \(20.70 \: In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs. Titration is often used to determine the concentration of a solution. Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample of known concentration.
from www.slideserve.com
Suppose that a titration is performed and \(20.70 \: \ce{naoh}\) is required to reach the end point when titrated. \ce{naoh}\) is required to reach the end point when titrated. Suppose that a titration is performed and \(20.70 \: In many cases it is not a simple matter to obtain a pure substance,. Titration is a technique of determining the concentration of unknown solution by using a solution of known concentration. The known solution (titrate) is added in. Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample of known concentration. Titration is often used to determine the concentration of a solution. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly.
PPT Ch 16 Redox Titrations PowerPoint Presentation ID6602548
Titration Concentration Formula Suppose that a titration is performed and \(20.70 \: Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample of known concentration. Titration is a technique of determining the concentration of unknown solution by using a solution of known concentration. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). \ce{naoh}\) is required to reach the end point when titrated. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly. Titration is often used to determine the concentration of a solution. Suppose that a titration is performed and \(20.70 \: In many cases it is not a simple matter to obtain a pure substance,. The known solution (titrate) is added in. Suppose that a titration is performed and \(20.70 \: \ce{naoh}\) is required to reach the end point when titrated.
From www.ck12.org
Titration (Calculations) Example 3 ( Video ) Chemistry CK12 Titration Concentration Formula Titration is a technique of determining the concentration of unknown solution by using a solution of known concentration. In many cases it is not a simple matter to obtain a pure substance,. The known solution (titrate) is added in. \ce{naoh}\) is required to reach the end point when titrated. Suppose that a titration is performed and \(20.70 \: Suppose that. Titration Concentration Formula.
From www.slideserve.com
PPT Calculating concentrations PowerPoint Presentation, free download Titration Concentration Formula In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly. Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample of known concentration. The known solution (titrate) is added in. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a. Titration Concentration Formula.
From missmeyerscience.blogspot.com
Miss Meyer's Science Site Titration Calculations Titration Concentration Formula Titration is a technique of determining the concentration of unknown solution by using a solution of known concentration. Suppose that a titration is performed and \(20.70 \: Titration is often used to determine the concentration of a solution. Suppose that a titration is performed and \(20.70 \: Let's assume you are titrating a strong acid (10 ml unknown concentration hcl). Titration Concentration Formula.
From fity.club
Titration Formula Titration Concentration Formula Suppose that a titration is performed and \(20.70 \: Titration is a technique of determining the concentration of unknown solution by using a solution of known concentration. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly. The known solution (titrate) is added in. Titration involves the gradual addition of a reagent of known concentration,. Titration Concentration Formula.
From www.wikihow.com
5 Easy Ways to Calculate the Concentration of a Solution Titration Concentration Formula In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly. Suppose that a titration is performed and \(20.70 \: \ce{naoh}\) is required to reach the end point when titrated. Titration is a technique of determining the concentration of unknown solution by using a solution of known concentration. Let's assume you are titrating a strong acid. Titration Concentration Formula.
From www.slideserve.com
PPT TITRATION PowerPoint Presentation, free download ID1459481 Titration Concentration Formula Suppose that a titration is performed and \(20.70 \: \ce{naoh}\) is required to reach the end point when titrated. In many cases it is not a simple matter to obtain a pure substance,. Titration is often used to determine the concentration of a solution. \ce{naoh}\) is required to reach the end point when titrated. Titration is a method to determine. Titration Concentration Formula.
From www.slideserve.com
PPT Unit 19 Acid Base Equilibria Titrations PowerPoint Presentation Titration Concentration Formula Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample of known concentration. The known solution (titrate) is added in. \ce{naoh}\) is required to reach the end point when titrated. Titration is a technique of determining the concentration of unknown solution by using a solution of known concentration. In a titration, 25.0. Titration Concentration Formula.
From courses.lumenlearning.com
AcidBase Titrations General Chemistry Titration Concentration Formula Suppose that a titration is performed and \(20.70 \: The known solution (titrate) is added in. \ce{naoh}\) is required to reach the end point when titrated. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs. Titration is often used to determine the concentration of a solution. In a. Titration Concentration Formula.
From mungfali.com
Acid Base Titration Procedure Titration Concentration Formula In many cases it is not a simple matter to obtain a pure substance,. Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample of known concentration. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). Suppose that a titration is. Titration Concentration Formula.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titration Concentration Formula In many cases it is not a simple matter to obtain a pure substance,. \ce{naoh}\) is required to reach the end point when titrated. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly. \ce{naoh}\) is required to reach the end point when titrated. Let's assume you are titrating a strong acid (10 ml unknown. Titration Concentration Formula.
From thescienceteacher.co.uk
Concentration and titrations teaching resources the science teacher Titration Concentration Formula \ce{naoh}\) is required to reach the end point when titrated. \ce{naoh}\) is required to reach the end point when titrated. Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample of known concentration. Suppose that a titration is performed and \(20.70 \: The known solution (titrate) is added in. Suppose that a. Titration Concentration Formula.
From www.wikihow.com
5 Easy Ways to Calculate the Concentration of a Solution Titration Concentration Formula Suppose that a titration is performed and \(20.70 \: \ce{naoh}\) is required to reach the end point when titrated. Titration is often used to determine the concentration of a solution. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly. Suppose that a titration is performed and \(20.70 \: \ce{naoh}\) is required to reach the. Titration Concentration Formula.
From mungfali.com
Acid Base Titration Calculation Titration Concentration Formula \ce{naoh}\) is required to reach the end point when titrated. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs. In many cases it is not a simple matter to obtain a pure substance,. Titration is a technique of determining the concentration of unknown solution by using a solution. Titration Concentration Formula.
From www.slideserve.com
PPT TITRATION PowerPoint Presentation, free download ID1459481 Titration Concentration Formula Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample of known concentration. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly. Titration is often used. Titration Concentration Formula.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Titration Concentration Formula Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample of known concentration. In many cases it is not a simple matter to obtain a pure substance,. Titration is often used to determine the concentration of a solution. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. Titration Concentration Formula.
From mungfali.com
Acid Base Titration Calculation Titration Concentration Formula Titration is often used to determine the concentration of a solution. In many cases it is not a simple matter to obtain a pure substance,. Suppose that a titration is performed and \(20.70 \: Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). Titration is a technique of determining. Titration Concentration Formula.
From yojkglpsgc.blogspot.com
How To Calculate Initial Concentration From Titration Curve Volume of Titration Concentration Formula Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved in a sample of known concentration. \ce{naoh}\) is required to reach the end point when titrated. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is exactly. The known solution (titrate) is added in. In many cases it is not a. Titration Concentration Formula.
From www.youtube.com
GCSE Chemistry Titration calculations worked examples YouTube Titration Concentration Formula Titration is a technique of determining the concentration of unknown solution by using a solution of known concentration. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs. \ce{naoh}\) is required to reach the end point when titrated. Titration is a method to determine the unknown concentration of a. Titration Concentration Formula.